請用 Wechat 掃描台灣精品QRcode 或 搜尋ID: TaiwanExcellence

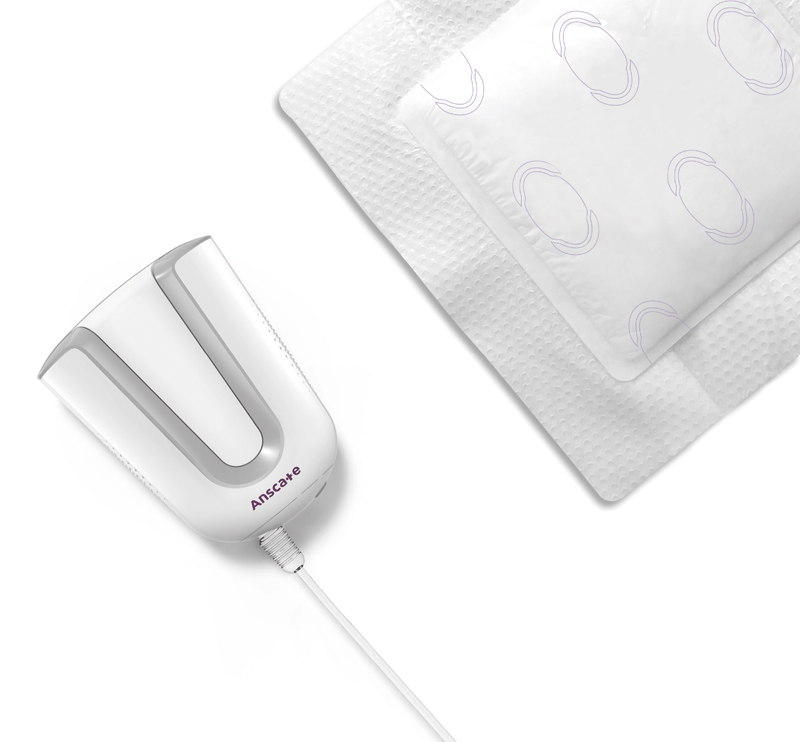

Anscare SIMO NPWT (Negative Pressure Wound Therapy) System can be used to promote wound healing in acute, chronic and burn wounds.
SIMO is lightweight and small, which can be put in patients’ pockets, providing a simple way to continue negative pressure therapy outside hospital with less expense.
Benefits
● Easy to use
● Promote blood circulation improvement and exudate management
● Simple and mobile
● Smart indicator for dressing change notification
● Display negative pressure status by color band and sensing knob
● Light showering is allowed
For more information, please visit Anscare website.
About BenQ Materials Corp.
Founded in 1998, BenQ Materials started with the development and manufacturing of optical storage products. Our ethos of “Live with Innovation” drives us forward in the pursuit of materials-science solutions through the cross-application of materials and six core manufacturing technologies. We leverage our expertise into developing four major application categories: functional films, advanced battery materials, medical, and fabrics. Today, BenQ Materials is a premier supplier of display-materials solutions and the proprietor of several leading brands in various industries. Headquartered in Taoyuan, Taiwan, the company has production sites in Taiwan (Taoyuan, Longtan, Yunlin) and China (Suzhou, Wuhu) as well as a number of sales and service offices in Taiwan, China and Malaysia.
As a leading manufacturer of medical sterilization packaging in Asia, SIGMA has 40+ years of industry know-how. Vertically integrated with BenQ Materials, we leverage the Group’s resources to propel innovations to new frontiers. We offer end-to-end services for medical device manufacturers, including material selection recommendations, comprehensive material testing, sterilization packaging, and final transportation. Our product verification and process validation procedures adhere strictly to ISO 11607 standards, and our lab quality management system complies with ISO 17025. Through rigorous sterile packaging inspection covering sterilization services, sterile barrier integrity, packaging strength, material properties, compatibility, and stability testing, we ensure our customers' peace of mind.
Contact info
Tel:03-3748800#2754
Fax:03-3619900#2754
Email:Penny.Tsai@BenQMaterials.com
Tel:03-3748800 #2882
Fax:03-3619900
Email:frances.peng@benqmaterials.com
Email:Richard.Neo@BenQMaterials.com
Tel:03-3748800#2881
Email:Jeannie.Cho@BenQMaterials.com
Tel:03-37488000#2754
Email:penny.tsai@BenQMaterials.com