請用 Wechat 掃描台灣精品QRcode 或 搜尋ID: TaiwanExcellence



Anscare LeniScar Silicone Stick is an easy-to-use topical silicone treatment intended to diminish hypertrophic scars and keloids characterized with a raised or discolored appearance. LeniScar creates an invisible, transparent thin film which helps to maintain the moisture balance of skin and provides an optimal protective barrier for the scar to heal.
Benefits
● A 12 hour long lasting moisturizing treatment
● Advanced silicone formula with Vitamin C & E
● Invisible, wearable under makeup
● Innovative scar management, no more messy gels or creams
● Ideal for scars on face, neck, joint, elbows, ankles and scar area covered with hair
For more information, please visit Anscare website.
About BenQ Materials Corp.
Founded in 1998, BenQ Materials started with the development and manufacturing of optical storage products. Our ethos of “Live with Innovation” drives us forward in the pursuit of materials-science solutions through the cross-application of materials and six core manufacturing technologies. We leverage our expertise into developing four major application categories: functional films, advanced battery materials, medical, and fabrics. Today, BenQ Materials is a premier supplier of display-materials solutions and the proprietor of several leading brands in various industries. Headquartered in Taoyuan, Taiwan, the company has production sites in Taiwan (Taoyuan, Longtan, Yunlin) and China (Suzhou, Wuhu) as well as a number of sales and service offices in Taiwan, China and Malaysia.
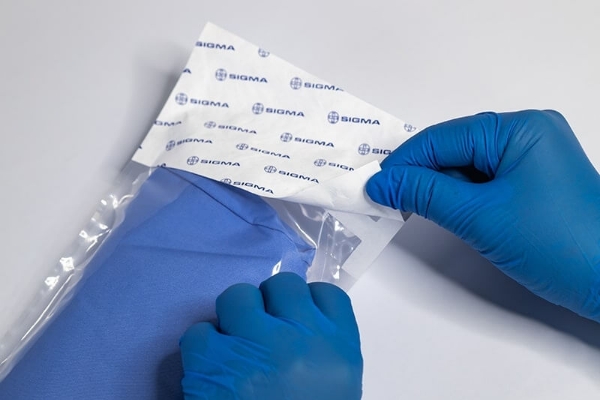
As a leading manufacturer of medical sterilization packaging in Asia, SIGMA has 40+ years of industry know-how. Vertically integrated with BenQ Materials, we leverage the Group’s resources to propel innovations to new frontiers. We offer end-to-end services for medical device manufacturers, including material selection recommendations, comprehensive material testing, sterilization packaging, and final transportation. Our product verification and process validation procedures adhere strictly to ISO 11607 standards, and our lab quality management system complies with ISO 17025. Through rigorous sterile packaging inspection covering sterilization services, sterile barrier integrity, packaging strength, material properties, compatibility, and stability testing, we ensure our customers' peace of mind.
Contact info
Tel:03-3748800#2754
Fax:03-3619900#2754
Email:Penny.Tsai@BenQMaterials.com
Tel:03-3748800 #2882
Fax:03-3619900
Email:frances.peng@benqmaterials.com
Email:Richard.Neo@BenQMaterials.com
Tel:03-3748800#2881
Email:Jeannie.Cho@BenQMaterials.com
Tel:03-37488000#2754
Email:penny.tsai@BenQMaterials.com