請用 Wechat 掃描台灣精品QRcode 或 搜尋ID: TaiwanExcellence


DermaAngel Acne Patch Plus(Non-Sterile) is an innovative acne patch that exclusively adds the salicylic acid to satisfy the consumer needs for shortening acne formation periods. To heal the acne faster by utilized the salicylic acid 's softening keratin capability and its antibacterial effect.
Besides, DermaAngel keeps developing the superior hydrocolloid formula to improve the shortages of the acne patch products on the market. Such as offer the highest absorption capacity acne patch which absorbs pus and oil effectively. And the moderate viscosity does not only allow the patch to be well-adhered to the skin but also prevents excessive stickiness to pull the skin when removed.
To solve the pain point that young consumers worry about the acne patch being too conspicuous and losing their confidence when using it. DermaAngel professionally develops the acne patch with the ultra-thin edge of 0.01cm designed and the nude matte surface to make it not reflective and invisible.
About BenQ Materials Corp.
Founded in 1998, BenQ Materials started with the development and manufacturing of optical storage products. Our ethos of “Live with Innovation” drives us forward in the pursuit of materials-science solutions through the cross-application of materials and six core manufacturing technologies. We leverage our expertise into developing four major application categories: functional films, advanced battery materials, medical, and fabrics. Today, BenQ Materials is a premier supplier of display-materials solutions and the proprietor of several leading brands in various industries. Headquartered in Taoyuan, Taiwan, the company has production sites in Taiwan (Taoyuan, Longtan, Yunlin) and China (Suzhou, Wuhu) as well as a number of sales and service offices in Taiwan, China and Malaysia.
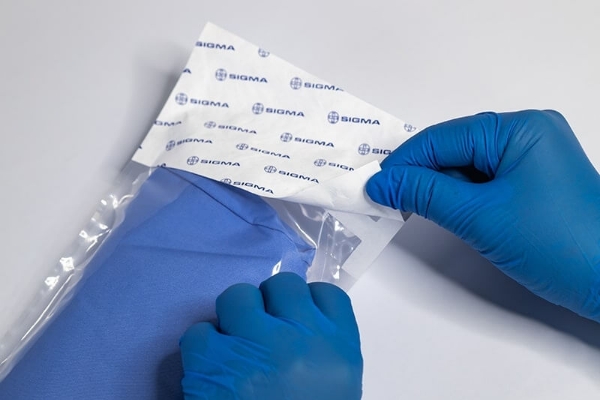
As a leading manufacturer of medical sterilization packaging in Asia, SIGMA has 40+ years of industry know-how. Vertically integrated with BenQ Materials, we leverage the Group’s resources to propel innovations to new frontiers. We offer end-to-end services for medical device manufacturers, including material selection recommendations, comprehensive material testing, sterilization packaging, and final transportation. Our product verification and process validation procedures adhere strictly to ISO 11607 standards, and our lab quality management system complies with ISO 17025. Through rigorous sterile packaging inspection covering sterilization services, sterile barrier integrity, packaging strength, material properties, compatibility, and stability testing, we ensure our customers' peace of mind.
Contact info
Tel:03-3748800#2754
Fax:03-3619900#2754
Email:Penny.Tsai@BenQMaterials.com
Tel:03-3748800 #2882
Fax:03-3619900
Email:frances.peng@benqmaterials.com
Email:Richard.Neo@BenQMaterials.com
Tel:03-3748800#2881
Email:Jeannie.Cho@BenQMaterials.com
Tel:03-37488000#2754
Email:penny.tsai@BenQMaterials.com