請用 Wechat 掃描台灣精品QRcode 或 搜尋ID: TaiwanExcellence


You're looking for a lens that gives you comfort from the time you step out of the house in the morning till you head home after a late night out with friends. Well, look no further.
With Miacare's revolutionary EautraSil™ Hydrophillic Silicone Technology setting a new benchmark in the industry with our high oxygen transmissibility, uncomfortable lens wear is a thing of the past! And to add to this, we look after your eyes in the long term too as our lenses help to ensure better corneal health!
Why settle for normal when you can have extraordinary? Ask for your Miacare SiHy lenses today!
EautraSil™ Hydrophilic Silicone Technology – A Revolutionary Breakthrough in Longer Wear Time
1. Ultra-High Oxygen Permeability Compared to Conventional Hydrogel Lenses
Conventional silicone hydrogel lenses are hydrophobic and require the use of solvents to combine silicone with hydrogel. To raise the standard of lens wear safety, miacare incorporates its world-leading EautraSil™ Hydrophilic Silicone Technology to modify silicone molecules with new hydrophilic properties so that the silicone and hydrogel can be combined without solvent treatment. This results in the world's first solvent-free formula for silicone hydrogel lenses: no irritation, no allergic reactions!
With oxygen transmissibility at Dk/t 150 , more oxygen is allowed to pass through the lenses quickly to the cornea, effectively preventing hypoxia-related complications, such as redness, corneal neovascularization and corneal epithelium-aging.
2. Solvent-free Formula – Irritation-free, Allergic Reaction-free, Improved Safety
The exclusive EautraSil™ Hydrophilic Silicone Technology, silicone molecules can be combined with hydrogel perfectly without the use of solvents, which could potentially leave residues that cause irritation to the eye and allergies.
3. Low Modulus at 0.6 MPa!– High Comfort
With hydrophilic silicone, less silicone is required to combine hydrogel. As a result, the low modulus affords softer, more comfortable wear.
4. Deposit-Resistant Sleek Lens Surface
The smooth water film on the lens surface prevents lipids and protein from depositing, ensuring the monthly use contact lens is always pristine clean, which keeps the vision bright and clear.
Benefits
● Ultra-high oxygen transmissibility at Dk/t 150
● Solvent-free formula!
● Smart peripheral edge design which helps to improve surface wettability, reducing irritation and providing extra comfort
● Lowest Modulus at 0.6 MPa! Offering a softer & more comfortable wear
● UV protection against the harmful rays of the sun
For more information, please visit Miacare website.
About BenQ Materials Corp.
Founded in 1998, BenQ Materials started with the development and manufacturing of optical storage products. Our ethos of “Live with Innovation” drives us forward in the pursuit of materials-science solutions through the cross-application of materials and six core manufacturing technologies. We leverage our expertise into developing four major application categories: functional films, advanced battery materials, medical, and fabrics. Today, BenQ Materials is a premier supplier of display-materials solutions and the proprietor of several leading brands in various industries. Headquartered in Taoyuan, Taiwan, the company has production sites in Taiwan (Taoyuan, Longtan, Yunlin) and China (Suzhou, Wuhu) as well as a number of sales and service offices in Taiwan, China and Malaysia.
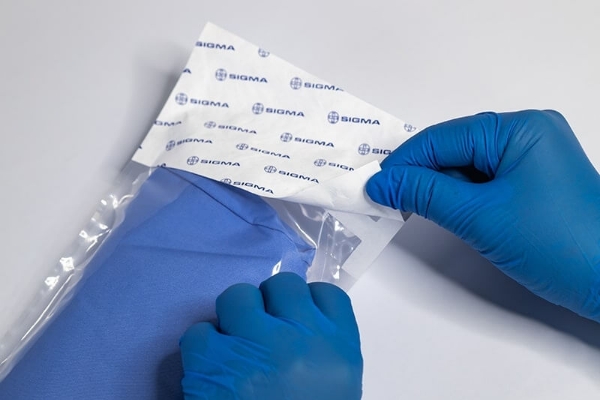
As a leading manufacturer of medical sterilization packaging in Asia, SIGMA has 40+ years of industry know-how. Vertically integrated with BenQ Materials, we leverage the Group’s resources to propel innovations to new frontiers. We offer end-to-end services for medical device manufacturers, including material selection recommendations, comprehensive material testing, sterilization packaging, and final transportation. Our product verification and process validation procedures adhere strictly to ISO 11607 standards, and our lab quality management system complies with ISO 17025. Through rigorous sterile packaging inspection covering sterilization services, sterile barrier integrity, packaging strength, material properties, compatibility, and stability testing, we ensure our customers' peace of mind.
Contact info
Tel:03-3748800#2754
Fax:03-3619900#2754
Email:Penny.Tsai@BenQMaterials.com
Tel:03-3748800 #2882
Fax:03-3619900
Email:frances.peng@benqmaterials.com
Email:Richard.Neo@BenQMaterials.com
Tel:03-3748800#2881
Email:Jeannie.Cho@BenQMaterials.com
Tel:03-37488000#2754
Email:penny.tsai@BenQMaterials.com